Laura Paxton
After years spent in sales, marketing, and strategy at Fortune 50 companies, I had the opportunity to create a company that reflected our values. Throughout the years we have developed highly configurable software to place formulary, patient safety, and healthcare worker safety (USP 800) information at the point of care. We believe that when we develop software in cooperation with our clients we arrive at the best and most consultative solutions to improve patient safety, medication safety, and employee safety.
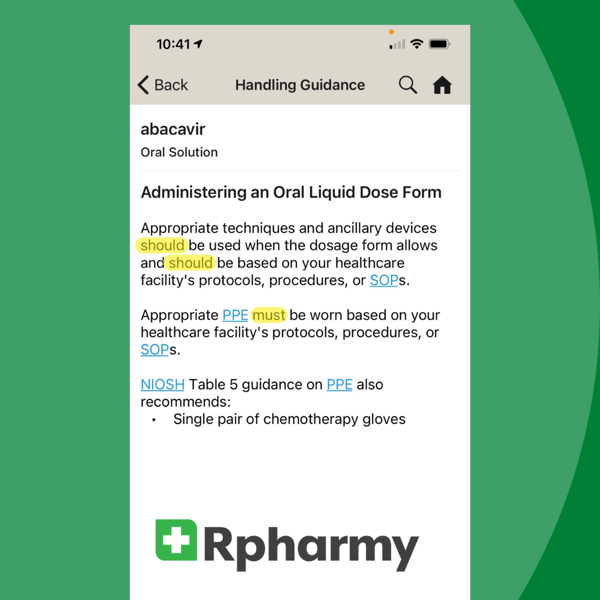
Spelling Out These Legal Terms in Hazardous Drugs Regulations
We're revisiting this blog first published in January 2021 because the terms 'should, shall, must' continue to cause confusion when trying to comply with FDA, OSHA, and USP <800> regulations. Are you one of many healthcare workers still searching for clear definitions of these tricky terms? We're here to help!
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
Are hazardous drug protocols documented and accessible in your facility? Seems like a no-brainer, especially considering all of the other potential upgrades and changes your facility may have to undertake to comply with USP <800>. But it’s among the most important pieces of the regulation because what good are policies if the healthcare workers they’re designed to protect do not know about them?
Read More
Topics:
Policy,
Formweb,
Formulary
We’re continuing to expand on the content from our most popular blog in 2021 - “From Our Customers: The Top 8 Questions To Expect In Joint Commission Inspections.” This time we’ll explain antimicrobial stewardship and how to prepare for the Joint Commission inspectors.
Read More
Topics:
Policy,
Formweb,
Formulary
The popularity of our most-read blog from 2021 - “From Our Customers: The Top 8 Questions To Expect In Joint Commission Inspections” - indicates that hospitals and healthcare systems continue to hustle to ensure all i’s are dotted and t’s are crossed before a visit from Joint Commission, accreditation or state board of pharmacy inspectors.
Read More
Topics:
Policy,
Formweb,
Formulary
The popularity of our most-read blog from 2021 - “From Our Customers: The Top 8 Questions To Expect In Joint Commission Inspections” - indicates that hospitals and healthcare systems continue to hustle to ensure all i’s are dotted and t’s are crossed before a visit from Joint Commission, accreditation or state board of pharmacy inspectors.
Read More
Topics:
Policy,
Formweb,
Formulary
Like many hospitals and healthcare systems, Duke University Hospital’s online formulary consisted of a spreadsheet housed on the intranet. The spreadsheet only listed medications that were considered formulary, and there was no information provided concerning associated restrictions, policies, or guidelines. This information was not readily available or easily retrievable for nurses and other staff members.
Read More
Topics:
Policy,
Formweb,
Formulary
Have you ever laid in bed at night and wondered “What will we do if our Designated Person leaves?” If this possibility hasn’t crossed your mind, it should.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
For Rpharmy’s state Department of Corrections (DOC) client, Formweb not only provides an online formulary solution that is easy to access and use, but also improves patient care by expanding communications about drugs in use to departments connected to but not directly part of the DOC.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
This blog has been updated since it was first published.
Early this year we heard accounts of citations around USP <797> requirements during discussions with healthcare systems and we are now hearing whispers that USP <797> will become fully enforceable around the end of this year. Because USP <797> and USP <800> are close cousins, when <797> becomes law, some portions of USP <800> around compounding will also be enforceable. Keep reading to ensure you are ready for the State Boards of Pharmacy, Centers for Medicare and Medicaid and accreditation inspectors.
The updated revision of USP <797> will move out of the final comments phase on March 31, 2022, making it that much closer to final. Once USP <797> is final it will also trigger some portions of USP <800> to be enforceable by the Joint Commission and state boards of pharmacy. In fact, we’re already hearing that citations will be issued starting as soon as February 2022 with fines coming down in August 2022. Your pharmacy or healthcare facility must be ready.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
We’re not joking or overpromising.
Assessment of Risk can be the biggest burden of USP <800> compliance. Once you’ve outlined your list of hazardous drugs and then determined which drugs will utilize alternative containment strategies and work practices, the work of gathering the AoR requirements begins.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb