Since the publication of “NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016” (NIOSH 2016), healthcare systems and facilities have been referring to Table 5 for hazardous drugs (HDs) safe handling guidance based on the activity and the formulation of the medication. Like the USP <800> mandate, the NIOSH 2016 directives in Table 5 were broad and provided general PPE instructions for the different types of HDs, including cancer chemotherapy, antivirals, and hormone therapies. In fact, NIOSH stated in the document: “This guidance does not cover all possible situations but provides general recommendations for the typical handling situations in healthcare.”
In May 2023, NIOSH released an updated document to expand on NIOSH 2016 Table 5 called “Managing Hazardous Drug Exposures: Information for Healthcare Settings.” In this additional guidance, NIOSH provides more in-depth HD assessment information and safety guidance throughout the document, including specific PPE and safe handling recommendations.
Notably, the new PPE safe handling table located in Chapter 8 is now organized by “Activity” and then “Formulation” vs. the reverse in NIOSH 2016. This new organization makes it simple to scan for the activity the healthcare worker will be involved in, like “disposal and cleaning,” and then determine the safety precautions needed based on formulation like “drug-contaminated waste.” The engineering controls are also clearly labeled and include Closed System Drug Transfer Device (CSTD) recommendations. In some activities and formulations, NIOSH has expanded on a previous “No” to say “No, unless a leak is observed or suspected,” for example.
While the new document doesn’t cover all exposure situations, it goes further to provide extensive risk management guidance and more context for various types of HDs and PPE required for everyday activities.
Integrating Chapter 8 into Your Organization
The Pharmacy can update PPE, engineering, and CSTD information in the formulary or hazardous drug list to provide more direction and protection to healthcare workers. The CDC cites that more than 8 million healthcare workers are exposed to HDs each year; how does your organization communicate the PPE required to protect healthcare workers from dangerous exposure?
Rpharmy provides a range of options for communicating PPE requirements, including simple text instructions such as 'Double chemotherapy gloves' or universally understood icons. Here’s an example of a facility that uses text and imagery to clearly communicate which PPE to use.
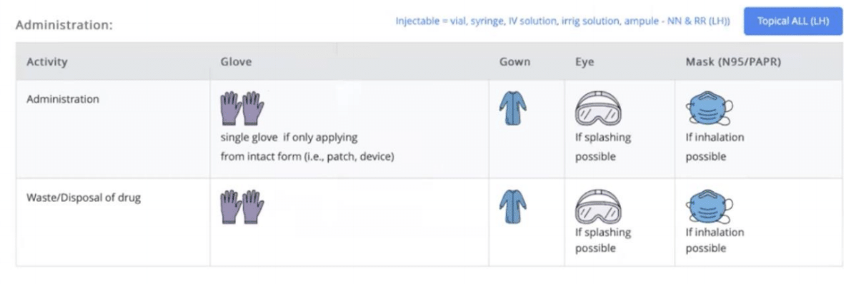
Here you can see that the images are supported by text.

In this image, PPE for Storage and Handling of various formulations is communicated with both images and text for clear communication.

To learn more about PPE needed to protect healthcare workers from hazardous drug exposure and comply with USP <800>, check out 8 Questions Answered About PPE and USP <800> in the Safety First Blog. If you’d like to learn more about Rhazdrugs, book a demo at your convenience here.


