Our Safety First blog on “Should, Shall and Must,” as used in the USP <800> hazardous drug safety requirements, continues to be one of our most searched-for and visited blogs. Therefore, we decided to write another blog exploring the topic in a little more depth to hopefully provide additional guidance on a topic that still causes confusion and concern.
USP <800> Compliance Challenges: Exploring the Gray Area between “Should” and “Must”
Leveraging Mobile Technology to Protect Healthcare Workers from Hazardous Drug Exposure
As you are well aware, ensuring safety in healthcare facilities goes beyond patient care; it also involves the important work of protecting healthcare workers from hazardous drug exposure. However, in an age of digital health and advanced technology, it's alarming that there are still healthcare professionals who resort to scouring the internet for safety information when dealing with hazardous drugs. This poses risks not only to their health but also to the overall safety standards of healthcare environments.
Achieving USP <800> Compliance: Expert Guidance from Rpharmy's Webinar Series
We know that since the USP <797> and USP <800> deadline was announced, November 1, 2023, has loomed large in the minds of hospital leaders, especially among pharmacy directors. In our many conversations with customers working to comply with the hazardous drug safe handling guidelines, we’ve learned most are still working towards compliance. In fact, in a Rpharmy survey, most did not feel ready for inspections around USP <800> guidelines.
USP <800> Compliance: Budgetary Challenges & Opportunities
More than 12 billion doses of hazardous drugs are handled per year, according to NIOSH. Each handling instance represents multiple opportunities for exposure - from the initial receiving of the drug to clinical administration and finally to safe disposal.
The Importance of Safe Hazardous Waste Disposal and Selecting a Waste Hauler
USP <800> establishes comprehensive protocols to ensure the safe handling of hazardous substances throughout their lifecycle, encompassing everything from initial reception and unpackaging by pharmacy staff to the administration by clinicians and final disposal in environmental services. The guidelines provide detailed instructions for disposal methods, ranging from basic waste disposal in designated containers to effectively managing incidents involving potentially hazardous spills, such as vomit.
Five Key Considerations: Is Your USP <800> Safe Handling Solution Truly Comprehensive?
USP <800> is set to be enforceable starting November 1, 2023, prompting hospitals and healthcare facilities to expedite their compliance plans. This entails updates to facilities, policies, and processes, along with effective communication to all healthcare workers exposed to hazardous drugs. However, ensuring that your USP <800> hazardous drugs safety solution is reliable can be a challenge. In this blog, we will discuss five essential attributes to consider when evaluating your USP <800> safe handling solution.
New NIOSH Expanded HD Safe Handling and PPE Guidance
Since the publication of “NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016” (NIOSH 2016), healthcare systems and facilities have been referring to Table 5 for hazardous drugs (HDs) safe handling guidance based on the activity and the formulation of the medication. Like the USP <800> mandate, the NIOSH 2016 directives in Table 5 were broad and provided general PPE instructions for the different types of HDs, including cancer chemotherapy, antivirals, and hormone therapies. In fact, NIOSH stated in the document: “This guidance does not cover all possible situations but provides general recommendations for the typical handling situations in healthcare.”
In May 2023, NIOSH released an updated document to expand on NIOSH 2016 Table 5 called “Managing Hazardous Drug Exposures: Information for Healthcare Settings.” In this additional guidance, NIOSH provides more in-depth HD assessment information and safety guidance throughout the document, including specific PPE and safe handling recommendations.
Notably, the new PPE safe handling table located in Chapter 8 is now organized by “Activity” and then “Formulation” vs. the reverse in NIOSH 2016. This new organization makes it simple to scan for the activity the healthcare worker will be involved in, like “disposal and cleaning,” and then determine the safety precautions needed based on formulation like “drug-contaminated waste.” The engineering controls are also clearly labeled and include Closed System Drug Transfer Device (CSTD) recommendations. In some activities and formulations, NIOSH has expanded on a previous “No” to say “No, unless a leak is observed or suspected,” for example.
While the new document doesn’t cover all exposure situations, it goes further to provide extensive risk management guidance and more context for various types of HDs and PPE required for everyday activities.
Integrating Chapter 8 into Your Organization
The Pharmacy can update PPE, engineering, and CSTD information in the formulary or hazardous drug list to provide more direction and protection to healthcare workers. The CDC cites that more than 8 million healthcare workers are exposed to HDs each year; how does your organization communicate the PPE required to protect healthcare workers from dangerous exposure?
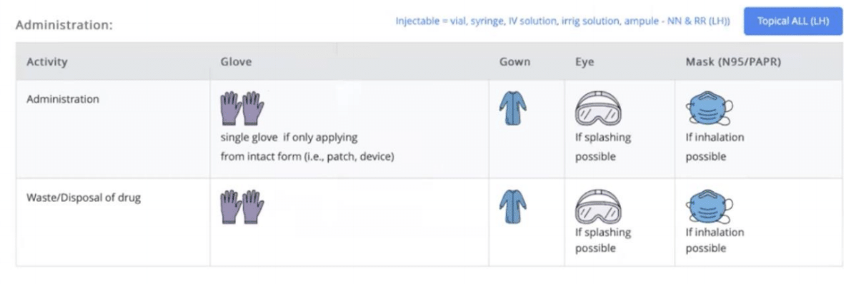
Rpharmy provides a range of options for communicating PPE requirements, including simple text instructions such as 'Double chemotherapy gloves' or universally understood icons. Here’s an example of a facility that uses text and imagery to clearly communicate which PPE to use.
It’s 2023, and healthcare systems are still only required to document and communicate safety guidelines for hazardous drugs on the NIOSH 2016 list … In the meantime, the NIOSH 2020 list is available but is still not the required point of reference for hazardous drug (HD) lists. However, NIOSH 2016 may soon be out and NIOSH 2020 may soon be the law of the land under the name: NIOSH List of Hazardous Drugs in Healthcare Settings 2023. The updated list will be official once the Federal Register reviews and approves it, although we still don't have an estimated date. Until then, we are still referencing NIOSH 2016.
How have you thanked a nurse recently? As the American Nurses Association (ANA) said, “The impact of nurses on healthcare is unparalleled, directly affecting the health and well-being of our society. Nurses are everywhere we live, work, play, learn, and worship, and in every healthcare setting, providing care to millions of people.”