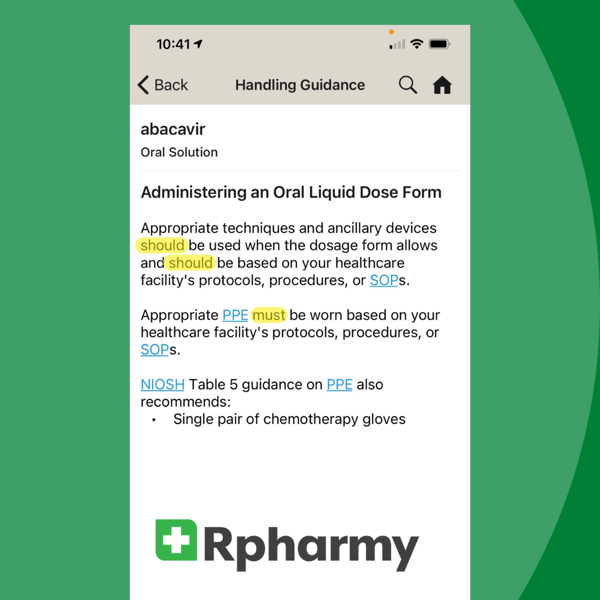
Spelling Out These Legal Terms in Hazardous Drugs Regulations
If you’re reading this, then you’re one of many healthcare workers who have second guessed the meanings of should, shall, and must in FDA, OSHA, and USP <800> regulations--or other legal documents as part of your work.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
It seems that you can't turn on the news without hearing about the next wave of COVID-19 that will soon travel across the U.S. Numbers are rising across the nation and there is again a great focus on the capacity of hospital systems to diagnose and treat sick patients, as well as maintain capacity for everyday operations outside of coronavirus care.
While much of the discussion is on the ability to care for the increase in patients, we can't help but focus on the safety of healthcare workers as well.
Read More
Topics:
Rhazdrugs,
USP <800>,
Technology
A shocking 8 million U.S. healthcare workers are potentially exposed to hazardous drugs (HDs) each year according to the CDC. In fact, a report in Pharmacy Times states, “compared with any other occupational setting, the health care setting uses the largest and most diverse array of agents that are hazardous to humans.” Not only are they exposed to a variety of hazardous agents every day, the effects may be compounded over the decades of their career.
Read More
Topics:
Rhazdrugs,
USP <800>,
Technology
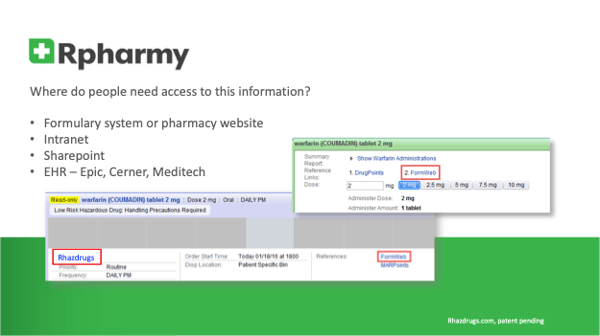
Rpharmy is raising the bar in USP 800 compliance with Rhazdrugs, a searchable hazardous drugs database accessible at the point of care improving visibility, frontline healthcare staff safety, and compliance with regulations including USP 800.
Read More
Topics:
Rhazdrugs,
USP <800>,
Technology
By Integrating Critical Information at Point of Care, Rhazdrugs Improves Frontline Healthcare Staff Safety, Raises Bar for USP 800 Compliance
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb,
News
Several months ago, in this same space, one of our clients shared her perspective around USP <800>. She discussed the complexity of managing the volumes of critical safety information and the continuous influx of updates, additions and changes. We felt it was a topic worth revisiting as we continue the conversation around the relationship between the cost and benefits of Rpharmy’s Rhazdrugs software.
Read More
Topics:
Rhazdrugs,
USP <800>
Online marketing 101 – if it takes someone more than three clicks to get somewhere, you’re going to lose a sale. After owning a SaaS-based company for more than 15 years, I wholeheartedly concur. But the stakes are much higher when those extra clicks compromise the safety of patients and their caregivers.
Read More
Topics:
Rhazdrugs,
Policy,
Technology,
Formweb
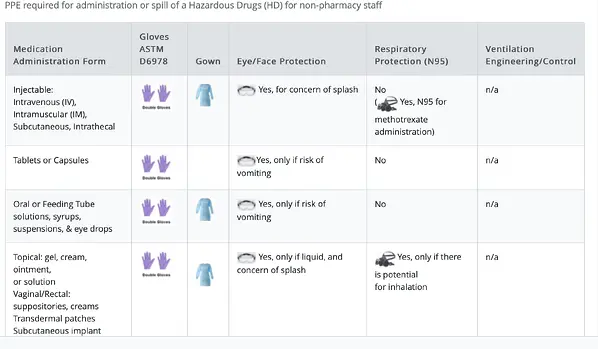
Last week we had the opportunity to explore the origins of PPE and the critical role it plays in keeping healthcare workers safe. This week, we thought we would talk about the costs related to PPE.
Read More
Topics:
Rhazdrugs,
COVID,
PPE
Personal Protective Equipment or PPE has been part of my vocabulary for nearly 30 years. But if I talked to someone outside of the healthcare industry about it prior to March 2020, they would have looked at me like I was speaking Greek. Today, PPE is one of the most ubiquitous three letter acronyms in the English language.
Read More
Topics:
Rhazdrugs