Spelling Out These Legal Terms in Hazardous Drugs Regulations
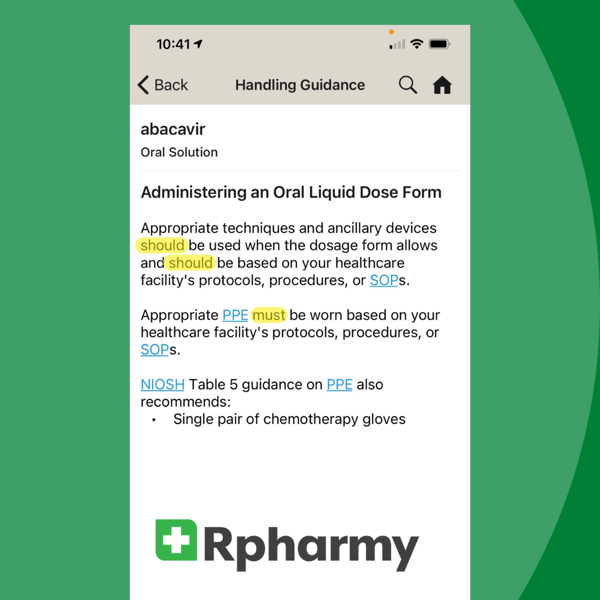
We're revisiting this blog first published in January 2021 because the terms 'should, shall, must' continue to cause confusion when trying to comply with FDA, OSHA, and USP <800> regulations. Are you one of many healthcare workers still searching for clear definitions of these tricky terms? We're here to help!
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
Have you ever laid in bed at night and wondered “What will we do if our Designated Person leaves?” If this possibility hasn’t crossed your mind, it should.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
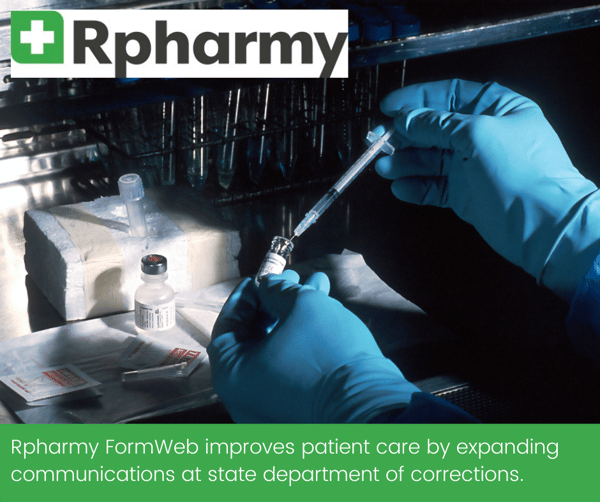
For Rpharmy’s state Department of Corrections (DOC) client, Formweb not only provides an online formulary solution that is easy to access and use, but also improves patient care by expanding communications about drugs in use to departments connected to but not directly part of the DOC.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
This blog has been updated since it was first published.
Early this year we heard accounts of citations around USP <797> requirements during discussions with healthcare systems and we are now hearing whispers that USP <797> will become fully enforceable around the end of this year. Because USP <797> and USP <800> are close cousins, when <797> becomes law, some portions of USP <800> around compounding will also be enforceable. Keep reading to ensure you are ready for the State Boards of Pharmacy, Centers for Medicare and Medicaid and accreditation inspectors.
The updated revision of USP <797> will move out of the final comments phase on March 31, 2022, making it that much closer to final. Once USP <797> is final it will also trigger some portions of USP <800> to be enforceable by the Joint Commission and state boards of pharmacy. In fact, we’re already hearing that citations will be issued starting as soon as February 2022 with fines coming down in August 2022. Your pharmacy or healthcare facility must be ready.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
As you are very aware the Epic electronic medical record system is making the transition from Hyperspace to Hyperdrive. Sorry (not sorry), but we have to say, “It will be Epic!”
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
We’re not joking or overpromising.
Assessment of Risk can be the biggest burden of USP <800> compliance. Once you’ve outlined your list of hazardous drugs and then determined which drugs will utilize alternative containment strategies and work practices, the work of gathering the AoR requirements begins.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
As we embark on a new year, we looked back at our most-read blogs and this one rose to the top. We're reposting because word is that USP <800> guidelines will be enforced in inspections this year. Hopefully, this information helps as you prepare for inspections this year. Also, be sure to browse through our blog library for more informative content around USP <800>, hazardous drugs and formulary.
A key way to protect healthcare workers is effective PPE, right? Seems simple enough except that USP <800> has outlined specific PPE requirements that vary depending on the NIOSH category of HD, how they are being handled, and in what part of the facility.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
It’s been another year of twists and turns, ups and downs, many lessons learned and deeper appreciations felt. We’ve watched our friends and clients in healthcare face challenge after exhausting challenge. With every obstacle, the healthcare industry continues to seek ways to care for the vulnerable and we are in awe.
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
Rpharmy Launches Rhazdrugs Platform for Hazardous Drug Handling, Disposal Safety Information at Point of Care
Leading Formulary Management, Drug Safety Information Provider Improves Healthcare Worker Safety by Providing Vital Safety Guidance In EHR, On Any Device
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb
Leading Formulary Management, Drug Safety Information Provider Improves Healthcare Worker Safety by Providing Vital Safety Guidance In EHR, On Any Device
Read More
Topics:
Rhazdrugs,
Policy,
USP <800>,
Formweb,
News