How are you handling the news that USP <797> and, therefore, USP <800> will be enforceable in less than a year?
Don't sleep on USP <800> like our sweet Lily here.
The “hurry up and wait” period is ending, and come this time next year, USP <797> and USP <800> will be enforceable by the Joint Commission, State Boards of Pharmacy and other accreditation entities.
Here's a month-long celebration we can get behind!
6 Factors For Identifying Hazardous Drugs in Your Organization
USP <800> requires healthcare organizations to document and communicate safety guidelines for hazardous drugs from the NIOSH hazardous drugs list. While this list is helpful, it is not comprehensive. Not only is the most current list more than two years old, but the FDA also approves hundreds of new drugs that could fall into the hazardous category each year.
USP <800> Wrap-Up: Policies and Procedures for Healthcare Worker Safety
Did you know that at least one hazardous drug policy or process made the Top Five USP <800> requirements being reviewed by State Boards of Pharmacy, Accreditors, and Centers for Medicare and Medicaid1?
Are Safety Data Sheets Up-to-Date, Available AND Being Used?
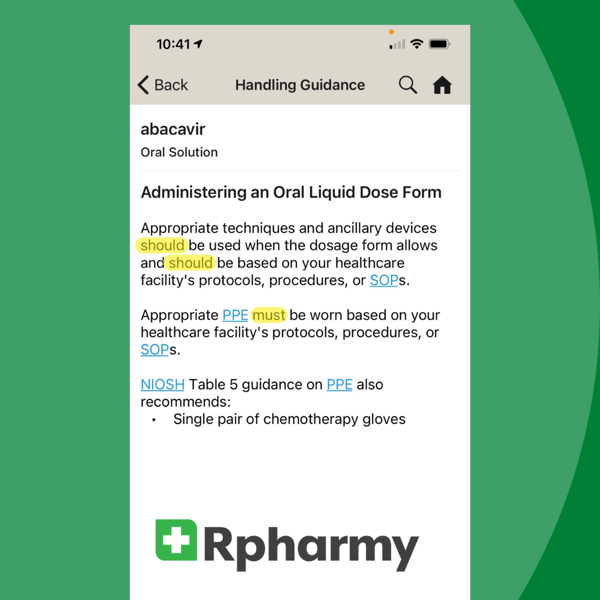
You can bet that the Joint Commission will be checking. We understand that locating, storing and communicating the Safety Data Sheets (SDS) is a laborious job. What if SDS were located on a central platform and, wait for it … were automatically available? It’s possible.
Spelling Out These Legal Terms in Hazardous Drugs Regulations
We're revisiting this blog first published in January 2021 because the terms 'should, shall, must' continue to cause confusion when trying to comply with FDA, OSHA, and USP <800> regulations. Are you one of many healthcare workers still searching for clear definitions of these tricky terms? We're here to help!
“If I leave, you’re sunk,” warns Designated Person
Have you ever laid in bed at night and wondered “What will we do if our Designated Person leaves?” If this possibility hasn’t crossed your mind, it should.
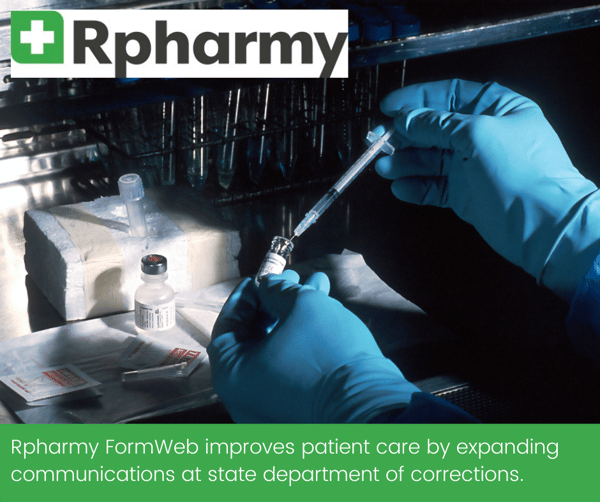
Formweb Broadly Expands Communication of Drug Information for a State Department of Corrections
For Rpharmy’s state Department of Corrections (DOC) client, Formweb not only provides an online formulary solution that is easy to access and use, but also improves patient care by expanding communications about drugs in use to departments connected to but not directly part of the DOC.
This blog has been updated since it was first published.
Early this year we heard accounts of citations around USP <797> requirements during discussions with healthcare systems and we are now hearing whispers that USP <797> will become fully enforceable around the end of this year. Because USP <797> and USP <800> are close cousins, when <797> becomes law, some portions of USP <800> around compounding will also be enforceable. Keep reading to ensure you are ready for the State Boards of Pharmacy, Centers for Medicare and Medicaid and accreditation inspectors.
The updated revision of USP <797> will move out of the final comments phase on March 31, 2022, making it that much closer to final. Once USP <797> is final it will also trigger some portions of USP <800> to be enforceable by the Joint Commission and state boards of pharmacy. In fact, we’re already hearing that citations will be issued starting as soon as February 2022 with fines coming down in August 2022. Your pharmacy or healthcare facility must be ready.